Wednesday 16 March 2011
uniqueness of snow flake
It is very unlikely for two snowflakes to be exactly alike due to the roughly 1019 water molecules which make up a snowflake,[8] which grow at different rates and in different patterns depending on the changing temperature and humidity within the atmosphere that the snowflake falls through on its way to the ground united.[9] Initial attempts to find identical snowflakes by photographing thousands of them with a microscope from 1885 onward by Wilson Alwyn Bentley found the wide variety of snowflakes we know about today.[10] It is more likely that two snowflakes could become virtually identical if their environments were similar enough. Matching snow crystals were discovered in Wisconsin in 1988. The crystals were not flakes in the usual sense but rather hollow hexagonal prisms.[11]
symmetry of snow flake
A non-aggregated snowflake often exhibits six-fold "radial" symmetry. The initial symmetry occurs because the crystalline structure of ice is six-fold. The six "arms" of the snowflake then grow independently, and each side of each arm grows independently. Most snowflakes are not completely symmetric. The micro-environment in which the snowflake grows changes dynamically as the snowflake falls through the cloud, and tiny changes in temperature and humidity affect the way in which water molecules attach to the snowflake. Since the micro-environment (and its changes) are very nearly identical around the snowflake, each arm grows in nearly the same way. Since the micro-environment of one snowflake is not the same as the micro-environment of a different snowflake, it is very unlikely that two snowflakes will be identical.
formation of snow flake
Snow crystals form when tiny supercooled cloud droplets (about 10 μm in diameter) freeze. These droplets are able to remain liquid at temperatures lower than −18 °C (0 °F), because to freeze, a few molecules in the droplet need to get together by chance to form an arrangement similar to that in an ice lattice; then the droplet freezes around this "nucleus." Experiments show that this "homogeneous" nucleation of cloud droplets only occurs at temperatures lower than −35 °C (−31 °F).[1] In warmer clouds an aerosol particle or "ice nucleus" must be present in (or in contact with) the droplet to act as a nucleus. The particles that make ice nuclei are very rare compared to nuclei upon which liquid cloud droplets form, however it is not understood what makes them efficient. Clays, desert dust and biological particles may be effective,[2] although to what extent is unclear. Artificial nuclei include particles of silver iodide and dry ice, and these are used to stimulate precipitation in cloud seeding.[3]
snow flake
Snowflakes are conglomerations of frozen ice crystals which fall through the Earth's atmosphere. They begin as snow crystals which develop when microscopic supercooled cloud droplets freeze. Snowflakes come in a variety of sizes and shapes. Complex shapes emerge as the flake moves through differing temperature and humidity regimes. Individual snowflakes are nearly unique in structure. Types which fall in the form of a ball due to melting and refreezing, rather than a flake, are known as graupel, with ice pellets and snow grains as examples of graupel
ice crystals
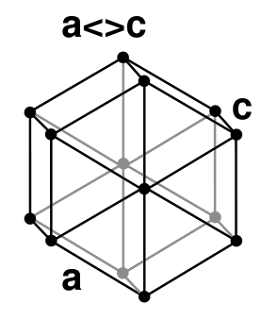
Ice crystals are a small crystalline form of ice including hexagonal columns, hexagonal plates, dendritic crystals, and diamond dust. The highly symmetric shapes are due to depositional growth, namely, direct deposition of water vapour onto the ice crystal. Depending on environmental temperature and humidity, ice crystals can develop from the initial hexagonal prism into numerous symmetric shapes. Possible shapes for ice crystals are columns, needles, plates and dendrites. If the crystal migrates into regions with different environmental conditions, the growth pattern may change, and the final crystal may show mixed patterns. An example are capped columns. Ice crystals tend to fall with their major axis aligned along the horizontal, and are thus visible in polarimetric weather radar signatures with enhanced (positive) differential reflectivity values. Electrification of ice crystals can induce alignments different from the horizontal. Electrified ice crystals are also well detectable by polarimetric weather radars.
Temperature and water vapor humidity determine crystalline forms. Ice crystals are responsible for various atmospheric optics displays.
Ice clouds are composed of ice crystals, the most notable being cirrus clouds and ice fog.
http://en.wikipedia.org/wiki/Ice_crystals
Temperature and water vapor humidity determine crystalline forms. Ice crystals are responsible for various atmospheric optics displays.
Ice clouds are composed of ice crystals, the most notable being cirrus clouds and ice fog.
http://en.wikipedia.org/wiki/Ice_crystals
student suggested tutorials
http://www.grasshopper3d.com/video/fractal-tree
for ice crystal,
this tutorial is very useful to create for ice crystal theme
for ice crystal,
this tutorial is very useful to create for ice crystal theme
Wednesday 9 March 2011
main theme- 5. veins in a leaf
simple,
main veins and small veins connect to each others seems very interesting
abstract shapes
all different shapes
main theme- 4. bubble
same pattern in different sizes - shows the variety of the shapes
very absract and interesting shapes
very absract and interesting shapes
Subscribe to:
Posts (Atom)